Cell Overview#
Plasma Membrane#
Outer shell of the cell responsible for seperating the cell from the enviornment
Regulates transport of nutrients and waste of the cell
Consists of a phospholipic bilayer (seperates hydrophobic materials on the interior of the membrane from the aqeous enviornment
The heads are hydrophilic, while the tails are hydrophobic
Lots of things are embedded in and pierce the bilayer (peripheral proteins, protein channels, Alpha-Helix proteins etc.)
The proteins which pierce the membrane can be asymetric (re: inside has one structure, outside has a different structure)
Cytoplasm#
refers to the intracellular content
80% of interior is water
consists of cytosol (intracellular fluid) and organelles (internal structures)
cytosol is a non-newtonian fluid and is colorless
Cytoskeleton#
Protein filaments in the cytoplasm
Holds cellular structure, aids in migration, signalling, and chromosome segregation during cytokinesis
There are more specialized cytoskeleton structures: flagella, cilia, filopodia, lammelipodia etc.
There are 3 board classes of cytoskeleton: actin, microtubules, and intermediate filaments. Actin are physically the smallest, microtubules are physically the largest
Cell shape dictated by microtubules main branches, with smaller actin branches doing fine shape
Organelles#
Nucleus#
Nucleus is present in eukaryotic cells
It’s enclosed by double membrane (re: two bilayers) supported by lamina (re: cytoskeleton support)
The outermost membrane folds onto itself into the endoplasmic reticulum (ER)
nuclear pores pieces into the interior of the nucleus
the interior is filled with chromatin (linear DNA molecules in complex with other proteins)
has other suborganelles (nucleolus/speckles)
Purpose:
stores genetic material
site of DNA replication and translation
ribosome synthesis
Endoplasmatic Reticulum#
Purpose:
lipid synthesis
membrane protein synthesis
Ca++ ion storage
detoxification
Network of interconnected closed membrane tubules and vesicles
composed of smooth ER and rough ER (called “rough” because it has ribosomes)
The rough ER helps transpose hydrophobic materials within their bilayers as well as ribosomes
The smooth ER generates new lipids and membranes
Golgi Apparatus#
Looks like the rough ER
Packages ER produces into small vesicles (exocytotic, secretory, lysosomal) which get transported to their final destination
Lysosomes#
single membrance vesicle which decomposes things
pH of 5
Peroxisomes#
Like lysosomes, but specifically fatty acids and toxic compounds
single membrance
They have a crystalline core
Mitochondrium#
drives ATP production for aerobic metabolism
has an double membrane, cristae, and a matrix
Chloroplast#
Photosynthesis site for plants
Plants also have mitocondria for some reason
Tissue Cultures#
The term “tissue culture” is used to denote a population of cells to be examined
“Culturing” a tissue means to grow them in a lab
Can be “In Vitro” (in a test tube /glass) or “In vivo” (in a living organism)
Depends on the subject of study
Why use cultures?
Controlled enviornment
Ease of access
Problems with tissue culture:
They are outside the body, so they aren’t a perfect replica of the system
Cells grow in 2D in vitro, while they grow in 3D in humans
Human cells divide roughly once a day in vitro, but rarely do in vivo
First proof of concept of tissue culture in 1885 by Wilhelm Roux was able to maintain chicken embryos in vitro for a couple of days
HeLa cells was the first cell culture line (1951)
From Henrietta Lack’s cervical cancer cells
Tissue Cultures are grown in dishes with a medium that has the correct nutrients, pH buffer ,indicator etc.
These dishes are stored in incubators
To work with these cells, you need to shove them into a sterile fume hood
Microscopy in Cell Biology#
Light Microscopy#
resolution of 200 nm
Oldest form of microscopy. Passes light through a thin section of cell tissue
More modern versions involve more lenses, but the same idea holds
For liquid sames, we need to invert the microscope (light coming from above, objective lenses below)
for many lense systems, you can insert “tube lenses” to circumvent the finite focal lengths of lenses
We can modulate the incoming light of a microscope to generate constrast
Humans are good at seeing changes in wavelength and amplitude, but bad at seeing phase and polarization changes
Most biological specimens respond to phase, so the trick is to convert the phase shifts to something that we can see
The idea is that:
you let light pass through your cells. Most will pass through just fine, but some will get phase shifted as they pass through things
Using a phase ring, you shift the diffracted light by a factor of $\frac{\pi}{4}$, leaving the phase of the unpeturbed light unchanged
Damp the amplitude of the unpeturbed light to the amplitude of the phase-modulated version
Look at the interference patterns between the two
Another method (Differential Interference Constrast Microscopy (DIC))
Send linearly polarized light through your sample
You have two equal polarization components
Use a Wollaston prism to seperate the two components away from each other
Pass the components through your specimen, which shifts the two polarizations a different amount
Recombine the components with a prism, then pass through an analyzer lens
Fluorescense Microscopy#
Idea: Shine one wavelength in, the dye you put into the cells reacts and produces a difference wavelength which you can see
The shift between the maximum of the emission and the absorption spectra is called the Stokes shift
You are limited to 4 colors:
Constrained to visible spectrum for obvious reasons
UV regime can be cyto-toxic to your cells
These is also some spread associated with each source
How do you make biological molecules fluorescent?
You get lucky and there is some compound which can naturally bind to the molecule you want to study
Phalloidin: a mushroom toxin that binds to actin
Mitotracker: synthetic binder which binds mitochondria
Design florescent anti-bodies which bind to the target structure
You permeate the cell (poke a bunch of holes into it), flush a bunch of these antibodies in and let them bind, and then flush a clean buffer solution to clear up the free floaters
obviously, this only works on dead cells
Direct chemical labelling for your Molecule of Interest
GFP (Green Florescent Protein)
Jellyfish proteins which can attached to living cell structures
Accomplished by modifying the genes which produce a protein of interest by attaching the genome of GFP in an appropriate place
Can attach to nearly any protein
No need for the microinjection with anti-bodies
Electron Microscopy#
Resolution of 1 nm
Uses DeBroglie wavelength of electron to increase the resolution compared to optics
Uses tunnelling to produce a modulating current which gets reconstructed to an image
Disadvantages:
Very high vacuum required
Specimens need to be fixed, embedded, sectioned an stained with an electron-dense material
Central Dogma of Molecular Biology#
DNA (deoxyribonucleic acid) to RNA (ribonucleic acid) via transcription
RNA to Protein via translation
DNA#
A polymer of highly charged polyelectrolytes
the monomers of DNA (nucleotides) are made of a phosphate group, a sugar, and an organic base
The sugar for DNA is deoxyribose, while for RNA it’s ribose
The organic bases are thymine (T), cytosine(C), adenine(A), guanine(G), and uracil(U)
T for DNA and U for RNA
T and A are purines (double ring structures) while C, G and U are pyrimadine (single ring)
A and T pair up for DNA (A and U for RNA), while C and G pair up in both
You match a purine and pyrimadine together to maintain the width of DNA
The pairing occurs via hydrogen bonding (A and T have 2 hydrogen bonds, while C and G have 3 hydrogen bonds)
When describing the double band structure, there is a 3’ and a 5’ end
The 3’ side is attached to an oxygen of a phosphate
The 5’ side is connected to the $CH_{2}$ side
The 3’ and 5’ sides have opposite polarity on each strand
There are 3 variants of DNA (A,B and Z)
DNA is not in isolation in the nucleus. They are complexed with histones
Since DNA is negatively charged from the phosphates, the histones are positively charged
The charge on the histones is modulated by the acetylation of its’ tails
RNA#
RNA is shares a lot of similarities to DNA
One big difference is that RNA has a flexible backbone
The three main types of RNA are messenger RNA, transfer RNA and ribosomal RNA
RNA Polymerase#
There are 3 polymerases which synthesis the RNA (RNA polymerase I synthesises rRNA. II for mRNA and II for tRNA)
These polymerases need a couple of things to do transcription:
A start sequence
The TATA box is a common one, but it’s not unique
Transcription factors are used to unwind and prepare the DNA
A stop sequence
a 5’ cap
The polymerase starts translating from the 3’ to the 5’ end (the strand that it works on is called the leading strand)
For mRNA, there are exon (protein encoding regions) and introns (non-protein encoding regions)
Proteins#
Consists of polymers
Each monomer has 3 components:
The amino group ($NH_{3}$)
The acid group ($CO_{2}H$)
A central carbon atom, which bridges the acid and amino groups (via single bond to N and C)
The R group: Gives all of the variance between the monomers
The monomers get chained together via peptide bonds
The process forms a single covalent bond between the carbon and nitrogen of the acid and amino group, producing water as a biproduct
The menagerie of Amino Acids side groups:
Nonpolar, aliphatic
Aromatic
Positively charged
Polar, uncharged
Negatively charged
There is a hierarchy to Proteins
Primary: the sequence of monomers
Secondary structure: $\alpha$ helices an $\beta$ pleated sheets
Tertiary structure: the 3D shape of your protein
Quaternary structure: multiple proteins complexing together
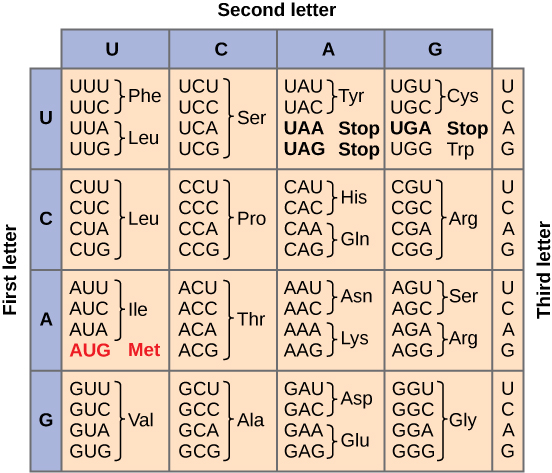
There is a well known mapping from RNA base pairs to monomers (re: codons)
Lipids#
Lipids are partitioned into polar heads and nonpolar tails
This partitioning allows lipid to self assemble (align the heads towards water and tails away from water)
There is also a whole zoo of lipids:
phospholipids
gangliosides
free fatty acids
Cholesterol
Steroids
Carbohydrates#
Vary in size: monosaccarides, oligosacharides, and polysacchardies
the number of rings determines which category they fall into
Consists of hydrogens and carbons (and some oxygens)
They can be energy sources, or serve a structural function (cellulose and chitin)
Mesoscopic Forces#
Van der Waals#
These are dipole-dipole interactions
There are 3 types:
between 2 permanent dipoles (Keesom orientation energy)
between 2 induced dipoles (London’s dispersion force)
between 1 permanent and 1 induced (Debye energy)
The interaction potential of a Van der Waals force between two point particles scales like $r^{-6}$
This does not hold for other geometries. You can derive other geometries via taking infinitesimal masses which do scale like $R^{-6}$
The proportionality constants come from quantum mechanic (Hamaker constant)
They are long range (> 10 nm)
They are weak (~ 1 $kJmol^{-1}$)
Hydrogen Bonds#
Occurs between a donor (strong polar group) and a proton acceptor (slightly electronegative)
They are weaker than ionic and covalent bonds (~ 10-40 $kJmol^{-1}$)
Used in molecular self-assembly
Hydration forces#
The polar nature of water leads to an electrostatic interaction
The large dipole moment of water + water’s capacity for hydrogen bonding gives rise to a short range-order of water molecules
Described by an exponential decay ($f = f_{0} exp(-\frac{t}{\lambda})$)
These are very short range (~1 nm)
Electrostatics#
point to point (Coulomb): scales like $r^{-2}$
point to dipole: scales like $r^{-3}$
dipole to dipole: scales like $r^{-3}$
the dominant long-range interaction
Debye-Hueckel Theory#
There is some screening of counter ions around the macro ion
The distribution of the counter ions as a function of the potential is $\rho_{i}(\phi) = \rho_{0} exp(-\frac{\phi}{kT})$
You can plug this charge distribution into the Poisson equation to yield: $\Delta \phi(\vec{r}) = \frac{1}{\epsilon_{0}\epsilon} \Sigma_{i=1}^{N} z_{i} e \rho_{i}(\infty) exp(-\frac{ - z_{i}e \phi(\vec{r})}{kT})$
DLVO Theory#
A Theory which describes forces between charged colloidal particles in a solution
$\frac{V(r)}{B} = -\frac{A}{12\pi r^{2}} + \frac{64 k T \epsilon_{0} \Gamma_{0}^{2}}{\kappa} exp(\kappa T)$
There is once again, competition between Van der Walls attraction and electrostatic repulsion
Depletion Forces#
Mediated by polymers or small particles
Imagine that you have an isotropic distribution of smaller particles (polymer chains for instance)
If you have two particles, then they feel an isotropic pressure from these particles
If these two particles are close enough (re: smaller than the average size of the polymer chains), then this will break the isotropy and push the particles together
generally attractive
driven by entropy gain of polymers
leads to colloidal aggregation
The joining pressure is $\Pi = \frac{N}{V} kT$
Undulation Forces#
Imagine that you have a very think membrane which you stretch out to sizes that are several orders of magnitude larger than the thickness
An energy of size kT is sufficient to substantially deform the system
Steric Forces#
mediated by polymers
The polymers act as a spring which repells particles away from a surface
The repulsive energy per unit area is $W(r) \approx 36 k T e^{-\frac{r}{R_{g}}}$
Bridging Flocculation#
The polymers get attached to colloids, and between two colloids, the polymers intertwine
Aggregating Self-Assembly#
Systems try to self-assemble so as to minimize $F_{min}$
errors in assembly can lead to disease:
sicle cell anemia
amyloidosis (prions)
Alzheimer’s disease (self-assembled beta sheets amyloidplaques)
Self-Assemblies:
aggregating (micellization of lipids)
non-aggregating (folding of globular proteins)
morphologies produced by phase separation
Self-Organization:
actively bringing/moving parts together (re mitosis)
Entropy loss of the assembling molecule is counteracted by entropy gain in solvent molecules
Self assemblies aim to minimize the surface energy, which runs contrary to Entropy maximization
Ostwald Ripening#
You have two particles which exchange particles via the solvent
This is a purely thermodynamic process
Surfactants#
they are ambiphiles (they have both hydrophilic and hydrophobic effects)
Phospholipids are an example of this type of molecules
These lipids tend to get attached to surfaces first prior to forming self assemblies
These phospholipids can get joined at their tails to self-assemble
Liquid Crystals#
Mesostate (between solid and liquid)
Anisotropic (preferred direction in behavior)
Ability to flow (liquid-like)
Lots of biological molecules for these crystals
biologically important (membranes, spider slik, cellulose etc.)
Roughly 4 types: Rod-like, disk-like, banana-like, and conical-like
Nematic: molecules tend to align in the same direction
Semetic: molecules tend to arrange themselves in layers
The order parameter $S = <P_{2}(\cos\theta)>$ determines which category the LC falls into
$\theta$ refers to the angle between the z-axis and the primary axis of the LC
$P_{2}$ is the 2nd order Legendre polynomial
Diffusion#
Brownian Motion#
Discovered by Brown in 1826 via pollen wiggling around
Einstein in 1905 gave kinematic formulation for Brownian motion
Random Walk#
You have a process described by:
$x_{i}(n) = x_{i}(n-1)_\epsilon$
On average, the displacement is 0
The mean squared displacement is non-zero ($n\epsilon^{2}$)
This can be related to the diffusion constant: $D = \frac{\epsilon^{2}}{2\tau}$
$tau$ is the time step of your simulation
On a macroscopic level, Fick’s Law states: $J_{x} = -D \frac{\partial c}{\partial x}$ J is the particle flux and c is the concentration
These concentrations are very dependent on the boundary conditions of your system